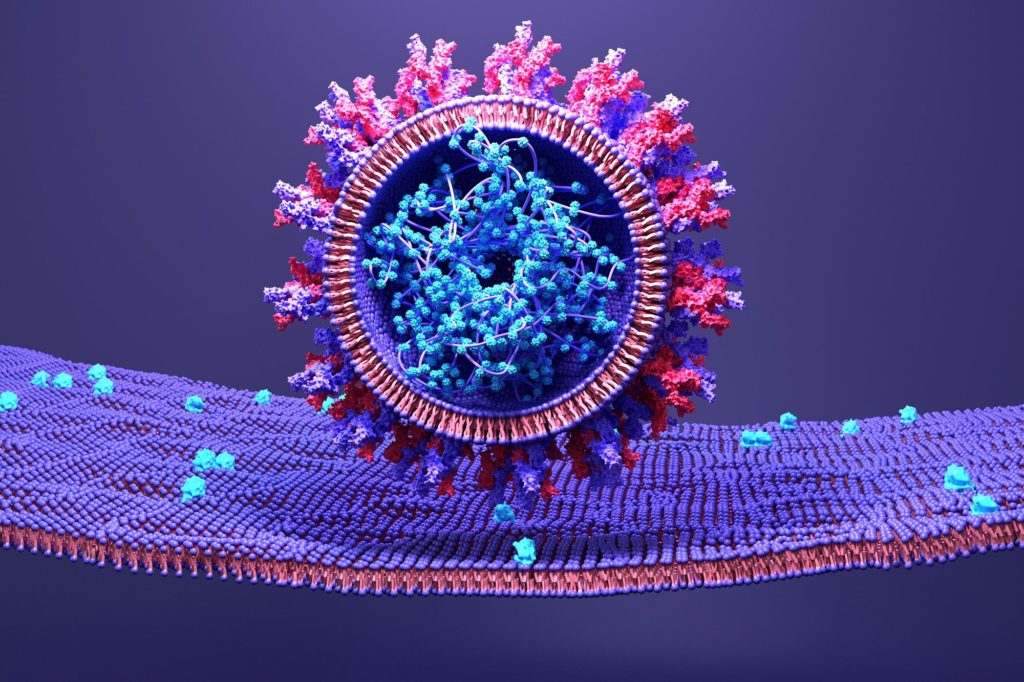
A structural model of the SARS-CoV-2 spike protein as the virus fuses with host human cells reveals an opportunity to disrupt dynamics and halt transmission.
Scientists have simulated the transition of the SARS-CoV-2 spike protein structure from when it recognizes the host cell to when it gains entry, according to a study published on August 31, 2021, in eLife.
The research shows that a structure enabled by sugar molecules on the spike protein could be essential for cell entry and that disrupting this structure could be a strategy to halt virus transmission.
An essential aspect of SARS-CoV-2’s lifecycle is its ability to attach to host cells and transfer its genetic material. It achieves this through its spike protein, which is made up of three separate components – a transmembrane bundle that anchors the spike to the virus, and two S subunits (S1 and S2) on the exterior of the virus. To infect a human cell, the S1 subunit binds to a molecule on the surface of human cells called ACE2, and the S2 subunit detaches and fuses the viral and human cell membranes. Although this process is known, the exact order in which it occurs is as yet undiscovered. Yet, understanding the microsecond-scale and atomic-level movements of these protein structures could reveal potential targets for COVID-19 treatment.
“Most of the current SARS-CoV-2 treatments and vaccines have focused on the ACE2 recognition step of virus invasion, but an alternative strategy is to target the structural change that allows the virus to fuse with the human host cell,” explains study co-author José N. Onuchic, Harry C & Olga K Wiess Professor of Physics at Rice University, Houston, US, and Co-Director of the Center for Theoretical Biological Physics. “But probing these intermediate, transient structures experimentally is extremely difficult, and so we used a computer simulation sufficiently simplified to investigate this large system but that maintains sufficient physical details to capture the dynamics of the S2 subunit as it transitions between pre-fusion and post-fusion shapes.”
The team was particularly interested in the role of sugar molecules on the spike protein which are called glycans. To see whether the number, type and position of glycans play a role in the membrane fusion stage of viral cell entry by mediating these intermediate spike formations, they performed thousands of simulations using an all-atom structure-based model. Such models allow you to predict the trajectory of atoms over time taking into account steric forces – that is, how neighboring atoms affect the movement of others.
The simulations revealed that glycans form a ‘cage’ that traps the ‘head’ of the S2 subunit causing it to pause in an intermediate form between when it detaches from the S1 subunit and when the viral and cell membranes are fused. When the glycans were not there, the S2 subunit spent much less time in this conformation.
The simulations also suggest that holding the S2 head in a particular position helps the S2 subunit recruit human host cells and fuse with their membranes, by allowing the extension of short proteins called fusion peptides from the virus. Indeed, glycosylation of S2 significantly increased the likelihood that a fusion peptide would extend to the host cell membrane, whereas when glycans were absent, there was only a marginal possibility that this would occur.
“Our simulations indicate that glycans can induce a pause during the spike protein transition. This provides a critical opportunity for the fusion peptides to capture the host cell,” concludes co-author Paul C. Whitford, Associate Professor at the Center for Theoretical Biological Physics and Department of Physics, Northeastern University, Boston, US. “In the absence of glycans, the viral particle would likely fail to enter the host. Our study reveals how sugars can control infectivity, and it provides a foundation for experimentally investigating factors that influence the dynamics of this pervasive and deadly pathogen.”
Reference: “Sterically confined rearrangements of SARS-CoV-2 Spike protein control cell invasion” by Esteban Dodero-Rojas, Jose N Onuchic and Paul Charles Whitford, 31 August 2021, eLife.
DOI: 10.7554/eLife.70362

Leave a Reply
You must be logged in to post a comment.