By FRONTIERS
Treatment with IFN-α2b was shown for the first time to improve virus clearance and decrease levels of inflammatory markers in a cohort of COVID-19 patients.
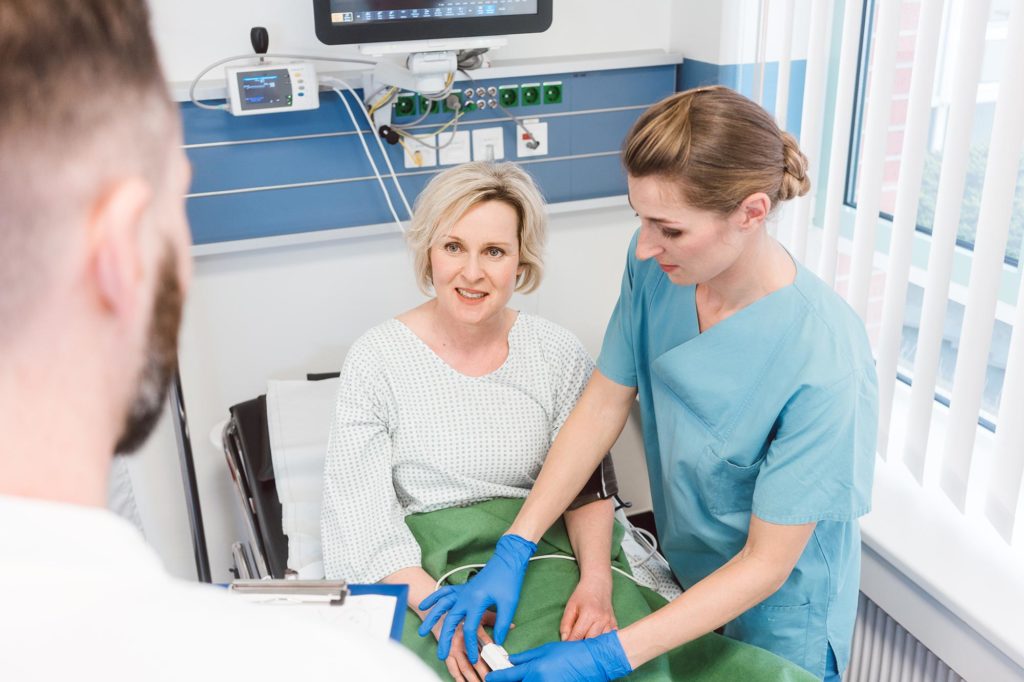
Treatment with antivirals such as interferons may significantly improve virus clearance and reduce levels of inflammatory proteins in COVID-19 patients, according to a new study in Frontiers in Immunology. Researchers conducting an exploratory study on a cohort of confirmed COVID-19 cases in Wuhan found that treatment with interferon (IFN)-α2b significantly reduced the duration of detectable virus in the upper respiratory tract and reduced blood levels of interleukin(IL)-6 and C-reactive protein (CRP), two inflammatory proteins found in the human body. The findings show potential for the development of an effective antiviral intervention for COVID-19, which is an ongoing global pandemic caused by the novel coronavirus, SARS-CoV-2.
“Interferons are our first line of defense against any and all viruses – but viruses such as corona-viruses have co-evolved to very specifically block an interferon response”, says lead author Dr. Eleanor Fish of the Toronto General Hospital Research Institute & University of Toronto’s Department of Immunology, adding: “This informs us of the importance of interferons for the clearance of virus infections. Treatment with interferon will override the inhibitory effects of the virus.”
Fish says that the research team considered IFN-α therapy for COVID-19 after they demonstrated interferons had therapeutic benefits during the SARS outbreak of 2002 and 2003. “My group conducted a clinical study in Toronto to evaluate the therapeutic potential of IFN-α against SARS. Our findings were that interferon treatment sped up the resolution of lung abnormalities in patients treated with interferon compared with those not treated with interferon” says Fish.
In this study, the authors examined the course of disease in a cohort of 77 individuals with con-firmed COVID-19 admitted to Union Hospital, Tongii Medical College, Wuhan, China, between January 16th and February 20th 2020. The individuals evaluated in this study consisted of only moderate cases of COVID-19, as none of the patients required intensive care or oxygen supplementation or intubation. Patients were either treated with IFN-α2b, arbidol (ARB), which is a broad-spectrum antiviral, or a combination of IFN-α2b plus ARB, and viral clearance was defined as two consecutive negative tests for virus at least 24 hours apart, from throat swab samples.
The researchers demonstrated a significantly different rate of viral clearance for each treatment group and notably, IFN-α2b treatment accelerated viral clearance by approximately 7 days. Treatment with IFN-α2b, whether alone or in combination with ARB, accelerated viral clearance when compared to ARB treatment alone. IFN treatment was also demonstrated to significantly reduce circulating levels of IL-6 and CRP, whether alone or in combination with ARB. The influence of age, co-morbidities and sex did not negate the effects of IFN treatment on viral clearance times or on the reduction in the inflammatory proteins IL-6 and CRP.
Despite the study’s limitations of a small, non-randomized cohort, the work provides several important and novel insights into COVID-19 disease, notably that treatment with IFN-α2b accelerated viral clearance from the upper respiratory tract and also reduced circulating inflammatory biomarkers, hinting at functional connections between viral infection and host end organ damage by limiting the subsequent inflammatory response in the lungs of patients.
Fish argues, “Rather than developing a virus-specific antiviral for each new virus outbreak, I would argue that we should consider interferons as the ‘first responders’ in terms of treatment. Interferons have been approved for clinical use for many years, so the strategy would be to ‘repurpose’ them for severe acute virus infections.”
As an uncontrolled, exploratory study, Fish says a randomized clinical trial is a crucial next step: “A clinical trial with a larger cohort of infected patients that are randomized to treatment with interferon-alpha or to a placebo would further this research”.
In the meantime, the findings from this study are the first to suggest therapeutic efficacy of IFN-α2b as an available antiviral intervention for COVID-19, which may also benefit public health measures by shortening the duration of viral clearance and therefore slowing the tide of the pandemic.
Reference: 15 May 2020, Frontiers in Immunology.
DOI: 10.3389/fimmu.2020.01061

Leave a Reply
You must be logged in to post a comment.